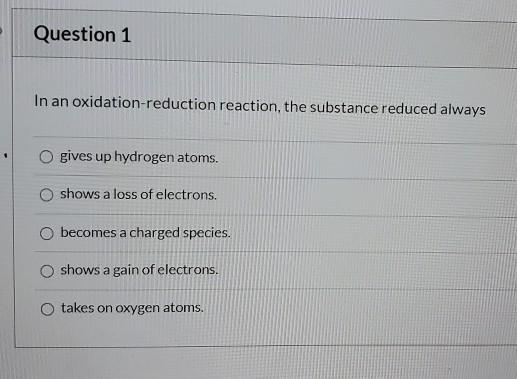
In an Oxidation Reduction Reaction the Substance Reduced Always
58 A gains electrons Bbecomes a charged speciesCtakes on oxygen atoms Dgives up hydrogen atomsEloses electrons. Reduction is the gain of electrons.
Solved Question 1 In An Oxidation Reduction Reaction The Chegg Com
The substance that is oxidized always acts as a reducing agent and the substance that is reduced always acts as the oxidizing agent.

. In which the oxygen number of O is II REDUCED from the zerovalent elemental state. Shows a gain of electrons. CH 4 g 2 O 2 g CO 2 g 2 H 2 Og.
It is only mentally that we can separate them. Shows a gain of electrons. We find examples of oxidation-reduction or redox reactions almost every time we analyze the reactions used as sources of either heat or work.
Formally a species that gains electrons is reduced and a species that loses electrons is oxidized. How do we do that. 1 e donor is always reduced and e acceptor is always oxidized state prior to reaction 2 the ability of a reaction to proceed depends on which half reactions are involved.
Becomes a charged species. E becomes a charged species. Gives up hydrogen atoms.
59In an oxidation-reduction reaction the substance reduced always ________. Reduction is the gain of electrons when. These are rare but the decomposition of hydrogen peroxide is an example.
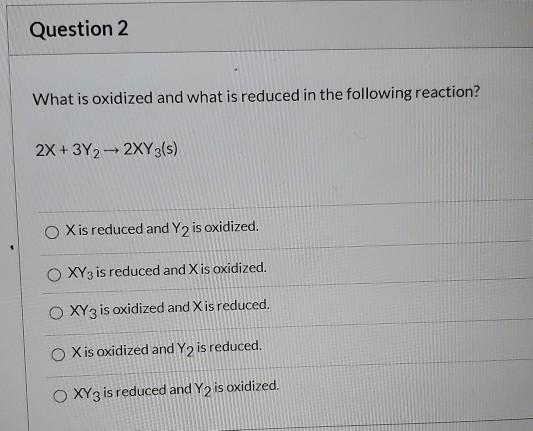
In an oxidation-reduction reaction the substance reduced always _____. Shows a loss of electrons. 59A loses electrons Bgives up hydrogen atoms Cgains electronsD.
Oxidation and reduction always occur together even though they can be. Question 1 In an oxidation-reduction reaction the substance reduced always O gives up hydrogen atoms. Oxidation is the loss of electrons.
O shows a loss of electrons. In an oxidation-reduction reaction the substance reduced always a takes on oxygen atoms. C gives up hydrogen atoms.
Neither reduction nor oxidation can occur separately. Takes on oxygen atoms. So oxidation and reduction always occur together.
Because electrons are neither created nor destroyed in a chemical reaction oxidation and reduction always occur in pairs it is impossible to have one without the other. Redox reactions require that we keep track of the electrons assigned to each atom in a chemical reaction. When oxygen typically is reacted with a hydrocarbon fuel the oxygen is formally reduced to CO_2 in which the oxygen number of O is -II REDUCED from the zerovalent elemental state.
D shows a gain of electrons. Substance oxidized H2 is electron donor while substance reduced O2 is electron acceptor. 28 One mole of particles of any substance contains how many particles.
So we write the individual reactions with the oxidation number of each constituent species superscripted. The OXIDANT is always reduced a redox reaction. In an oxidation-reduction reaction the substance that is reduced always shows a loss of electrons.
When oxygen typically is reacted with a hydrocarbon fuel the oxygen is formally reduced to CO2. Chemical reactions that involve the transfer of electrons are called oxidation-reduction or redox reactions. Gives up hydrogen atoms.
Takes on oxygen atoms. 25 In an oxidation-reduction reaction the substance reduced always D shows a gain of electrons. The OXIDANT is always reduced in a redox reaction.
In reality oxidation and reduction always occur together. O takes on oxygen atoms. Redox reactions require that we keep track of the electrons assigned to each atom in a chemical reaction.
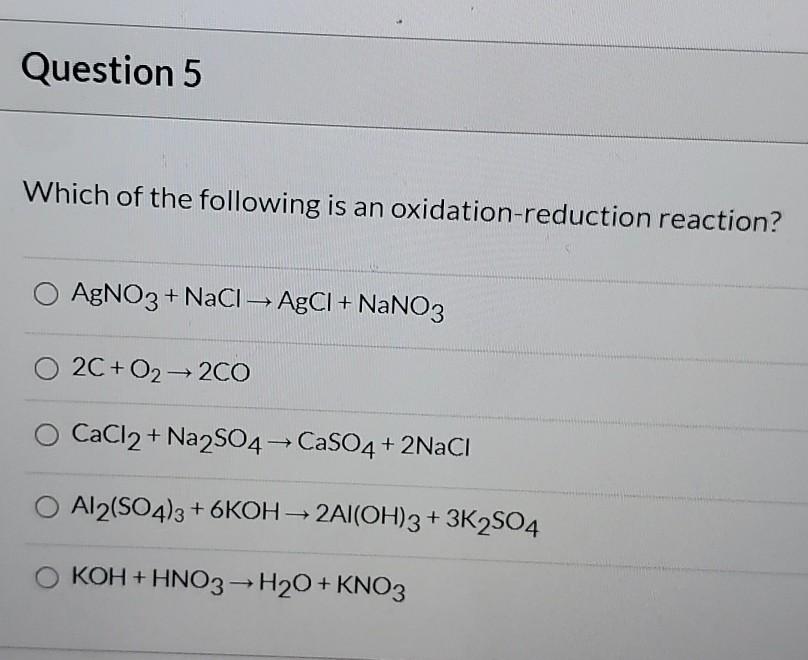
58In an oxidation-reduction reaction the substance oxidized always ________. Chemistry questions and answers. For this reason these reactions are called Redox Reactions.
Becomes a charged species c. Becomes a charged species. Group of answer choices the amount of solute does not change the amount of solvent does not change there is more solute in the concentrated solution the volume of the solution does not change water is removed from the concentrated solution.
Up to 256 cash back In an oxidation-reduction reaction the substance reduced always. Thus it is clear that if one substance is oxidised it must reduce the other substance. It shows that both oxidation and reduction always go side by side.
100 M 150 M 400 M Question 6 6. In an oxidation-reduction reaction bartleby. When natural gas burns for example an oxidation-reduction reaction occurs that releases more than 800 kJmol of energy.
There are reactions disproportionation reactions where one substance can act as both a reducing agent and oxidizing agent. In an oxidation-reduction reaction the substance oxidized always _____. A change occurs in the number of electrons.
The oxidized element is the ironically referred to as the reducing agent. Formally a species that gains electrons is reduced and a species that loses electrons is oxidized. Redox reactions involve formal electron transfer and a FORMAL change in oxidation number.
Chemical reactions that involve the transfer of electrons are called oxidation-reduction or redox reactions. Image Zerovalent copper is OXIDIZED to C u 2 and A g is reduced to silver metal A 0 g. Takes on oxygen atoms.
A redox reaction is a reaction where reduction and oxidation occur simultaneously. During the process of diluting a solution to a lower concentration _____. Shows a gain of electrons.
Becomes a charged species. In the word Redox the word red refers to reduction and ox refers to oxidation. It is only mentally that we can separate them.
Oxidation is the loss of electrons when electrons are added to the product. How do we do that. During oxidation a substance loses one or more electrons.
Chemical reactions in which electrons are transferred are called oxidation-reduction or redox reactions. When electrons are gained by one substance the electrons must come from another substance which loses the electrons. In the below reaction Magnesium gets oxidized by losing two electrons to oxygen which gets reduced by accepting two electrons from magnesium.
During reduction a substance gains one or more electrons. B shows a loss of electrons. Obecomes a charged species.
Solved Question 1 In An Oxidation Reduction Reaction The Chegg Com

Solved Question 3 In An Oxidation Reduction Reaction The Chegg Com

Solved In An Oxidation Reduction Reaction The Substance Chegg Com
Solved Question 1 In An Oxidation Reduction Reaction The Chegg Com
No comments for "In an Oxidation Reduction Reaction the Substance Reduced Always"
Post a Comment